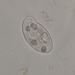
Φωτογραφίες / ήχοι
Παρατηρητής
douchΠεριγραφή
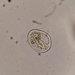
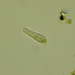
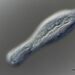
Bright-field microscopy. Between barbs, on internal surface of detached Trichoglossus moluccanus moluccanus feather.
Image 1: body
Image 2: anterior; upper focal-plane
Image 3: anterior; lower focal-plane
Image 4: posterior; upper focal-plane
Image 5: posterior; lower focal-plane
Image 6: feather
Φωτογραφίες / ήχοι
Παρατηρητής
peptolabΠεριγραφή
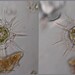
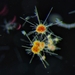
Plagiopyla ovata Kahl, 1931 from the fine sand sulfidic intertidal benthos of marine estuary Acabonac Harbor at Landing Lane. Imaged in Nomarski DIC on Olympus BH2S using SPLANAPO 40 0.95 objective and SPLAN 100 1.25 oil objective with slide oiled to condenser plus variable phone camera cropping on Samsung Galaxy S9+.
Several individuals are shown which range from 64 up to 80 um in length. The cell size and upward curving mid-region of the oral tube with the right side of upper oral lip slightly bulged forming a nose-like structure serve to distinguish the species from its closest congener P. frontata.
"Body size in vivo about 50–85×33–48µm; length to width ratio about 1.5–2.0:1 in vivo and 1.4–1.9:1 in stained specimens. Body rigid, non-contractile, dorsoventrally flattened, obovate, anterior end widely rounded, posterior end more or less narrowed (Figs 3a, d and 4a–c). Single macronucleus, globular to ellipsoidal, centrally located, about 20–30×15–24µm in vivo and 17–29×12–24µm in stained specimens (Figs 3g and 4d, j). Single micronucleus, globular, about 5µm in diameter in vivo and 4µm in diameter after protargol impregnation, closely associated with macronucleus, i.e. located at edge of, or slightly enveloped by, macronucleus (Figs 3g and 4j). Extrusomes slightly curved, 4–8µm long, distributed uniformly in ectoplasm and randomly in endoplasm (Figs 3a, e and 4e); extruded extrusomes slender needle-like (Figs 3e and 4k). Single contractile vacuole located near posterior end of cell, with two distinct pores located behind dense ciliary rows, pores observable in both living and stained specimens (Figs 3a, b, i and 4d, e)" (1).
"Striated band on dorsal side, starting at level of buccal cavity and extending anteriorly a short distance, then turning sharply backwards forming a loop about 7–10µm long, extending posteriorly parallel to somatic kineties, and terminating just below mid-body region (Figs 3b, i and 4f, i, o). Distance from anterior end of cell to beginning of striated band about 25% of cell length. In total, striated band about 34µm long, i.e. approximately half of cell length, consisting of numerous transverse ridges, each 2–3µm wide. Locomotion by swimming while rotating about main body axis, always spiralling to left" (1).
"In total, 56–63 monokinetidal somatic kineties (30–37 on ventral side and 23–30 on dorsal side), kinetids of ventral kineties usually more densely packed than those of dorsal kineties (Figs 3h, i and 4n, o). One or two dense ciliary rows (DC) in posterior region of cell on dorsal side left of cytoproct, about 6–21µm long, composed of 13–22 kinetosomes (for individuals with two DCs, only the left one is counted) (Figs 3b, i and 4o). Somatic cilia 6–10µm long, uniformly and densely distributed on cell surface (Figs 3a, h, i and 4a, n, o). About ten caudal cilia located along left posterior margin of cell, 8–10µm long, straight and oriented in a different direction to somatic cilia (Fig. 3a). Silverlines in irregular-rectangular grid (Figs 3c and 4i)" (1).
"Oral region formed by two lip-like structures, composed of dense oral kineties. Right side of upper oral lip slightly bulged forming a nose-like structure (Figs 3a and 4h). Slit-like buccal opening located at right-ventral side of cell (Figs 3a, h and 4a). Distance from anterior end of cell to beginning of buccal opening about 25% of cell length (Figs 3a and 4a–c). Tube-like buccal cavity about 85% of cell width, extending transversely to left and curving upwards in mid-region (Figs 3a, f and 4a–d, h). Distance from anterior end of cell to top of buccal cavity about 6–9µm. Oral ciliature more dense than somatic ciliature (Figs 3h and 4n). 53–61 oral kineties on cell surface and extending inwards (Figs 3f, g and 4m, n). Upper oral lip kineties 2–4µm long. Gap between upper oral lip kineties and somatic kineties about 2–3µm (Figs 3a, h and 4g, l, n)": (1).
"The genus Plagiopyla includes nine marine species viz., Plagiopyla binucleata Agamaliev, 1978, Plagiopyla cucullio (Quennerstedt, 1867) Wallengren, 1918, P. frontata Kahl, 1931, Plagiopyla marina Gourret & Roeser, 1886, Plagiopyla minuta Powers, 1933, P. mystax (Lynch, 1930) Nitla et al., 2019, Plagiopyla nyctotherus Poljansky & Golikova, 1959, P. ovata Kahl, 1931, and Plagiopyla stenostoma Alekperov & Asadullayeva, 1996. Among these, P. minuta, P. nyctotherus, and P. mystax are endocommensals of the sea urchin Strongylocentrotus, whereas other species are free-living. Most species of the genus Plagiopyla (including P. ovata) have a single ovoidal to ellipsoidal macronucleus and a single spherical micronucleus. However, two of the known marine species have different nuclear characteristics, i.e. two macronuclei in P. binucleata, and one vermiform macronucleus in P. stenostoma, and thus can be easily distinguished from their congeners" (1).
"P. frontata differs from P. ovata in having a shorter frontal region section (region between anterior cell margin and upper oral lip) relative to the length of the cell (17% in P. frontata vs. 25% in P. ovata), a straight buccal cavity tube (vs. a buccal tube curving upwards in its mid-region in P. ovata), and the upper oral lip perpendicular to the buccal opening (vs. not perpendicular in P. ovata). P. marina differs from P. ovata in having a shorter frontal region relative to the length of the cell (17–20% in P. marina vs. 25% in P. ovata) and long ovoid (vs. ovate to obovate) body shape" (1). P. ovata is smaller than P. frontata. Nitla et al. 2019 emphasized the size range reported by Kahl of P. frontata versus P. ovata 113–174 um vs 73–79 um (2).
- Redescription and SSU rRNA gene-based phylogeny of an anaerobic ciliate, Plagiopyla ovata Kahl, 1931 (Ciliophora, Plagiopylea). Ran Li, Wenbao Zhuang, Congcong Wang, Hamed El-Serehy, Saleh A. Al-Farraj , Alan Warren and Xiaozhong Hu. Int. J. Syst. Evol. Microbiol. 2021;71:004936 DOI 10.1099/ijsem.0.004936
- Critical revision of the family Plagiopylidae (Ciliophora: Plagiopylea), including the description of two novel species, Plagiopyla ramani and Plagiopyla narasimhamurtii, and redescription of Plagiopyla nasuta Stein, 1860 from India VENKATAMAHESH NITLA, VALENTINA SERRA, SERGEI I. FOKIN, LETIZIA MODEO, FRANCO VERNI, BHAGAVATULA VENKATA SANDEEP, CHAGANTI KALAVATI and GIULIO PETRONI. Zoological Journal of the Linnean Society, 2019, 186, 1–45.
Φωτογραφίες / ήχοι
Τι
Γαστερόποδα (Ομοταξία Gastropoda)Παρατηρητής
zihaowangΠεριγραφή
800x and 200x in sea water. It has a spiral shell. The cilia on the two wheels were actively moving.
Παρατηρητής
karolinaΠεριγραφή
L: 195, W: 5.5 um, apex ca. 4.5
8 pyrenoids/semicell, barely tapering towards apex
fits pretty well though a little more slender than usual. Noted as a fairly rare species, typical for bogs and soft waters. Known from Quebec.
Φωτογραφίες / ήχοι
Παρατηρητής
wrecklessmarineΗμερομηνία
Φεβρουάριος 2022Τόπος
Λείπει Η ΤοποθεσίαΠεριγραφή
68m
Φωτογραφίες / ήχοι
Παρατηρητής
mnold1Περιγραφή
Mag 100x and 400x
Not certain of the ID. Intense red color in the corona. (?) - Compare to https://www.plingfactory.de/Science/Atlas/KennkartenTiere/Rotifers/01RotEng/source/Keratella%20serrulata%20curvicornis.html.
- A water sample was taken on 4/26/2023, from the shore of a Pine Swamp impoundment, using a 10µ dip net to enrich for microbes. Air temp. 58°F.
Φωτογραφίες / ήχοι
Παρατηρητής
jane_trembathΠεριγραφή
Several specimens of fish lice crawling out from the scales of a dying carp that I removed from the water.
Picture at different focal lengths show the suckers as well as various hooks for attaching themselves to the host.
Ranged in size from 3-5mm.
Φωτογραφίες / ήχοι
Τι
Σκνίπες (Οικογένεια Ceratopogonidae)Παρατηρητής
microvisumΠεριγραφή
Проба воды из болотца. Water sample from the swamp
Φωτογραφίες / ήχοι
Παρατηρητής
kenkΠεριγραφή
Marine specimen from the Monterey Bay. While viewing the specimen it detached from its lorica and swam away. The GIF of it swimming is 50% speed.
Φωτογραφίες / ήχοι
Παρατηρητής
b_harveyΠεριγραφή
Moniliform macronucleus, longitudinal row of contractile vacuoles, dark parapharyngeal granular masses at posterior end
Φωτογραφίες / ήχοι
Παρατηρητής
franj36Περιγραφή
Freshwater
Semipolarized light.
40 X + smartphone zoom.
Φωτογραφίες / ήχοι
Παρατηρητής
douchΠεριγραφή
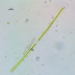
Bright-field light microscopy; scanning-power. In algae at edge of slow-flowing river. ~1 cm long when extended.
Image 1: anterior
Image 2: middle of body
Image 3: posterior
Τι
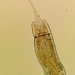
Ασκομύκητες (Συνομοταξία Ascomycota)Παρατηρητής
douchΗμερομηνία
Ιανουάριος 3, 2023Περιγραφή
Bright-field light microscopy; low-power. In air.
Φωτογραφίες / ήχοι
Παρατηρητής
peptolabΠεριγραφή
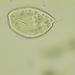
Phacus longicauda from the edge benthos of the acidic freshwater pond Chatfield's Hole situated in a white pine forest. This is the type species (holotype) of the genus Phacus. Length 175 um including the tail.
Species: 120-170 μm long, 45-70 μm wide; usually slightly twisted; a long caudal prolongation; flagellum about 1/2 body length; stigma prominent; a discoidal paramylon (paramylum) body central; pellicle longitudinally striated (Kudo, 1966).
var. longicauda: cell body 85-170 μm long, 45-70 μm wide; locomotive flagellum shorter than the cell body; a single paramylon body ring shaped (An Illustrated Guide to Freshwater Zooplankton in Japan, 2000).
Phacus longicauda (Ehrenberg) Dujardin 1841: 337, pl. 5 fig. 6
Published in: Dujardin, F. (1841). Histoire naturelle des Zoophytes, Infusoires, comprenant la physiologie et la clasification de ces animaux et la manière de les étudier à l'aide du microscope. pp. i-xii, 1-684. Paris: Librarie Encyclopédique de Roret
Φωτογραφίες / ήχοι
Παρατηρητής
dgborinΠεριγραφή
My first attempt at diatoms cleaning.
Three specimens observed, from the same sample of my previous observation https://www.inaturalist.org/observations/141202070 taken on 2022-11-05.
Stria density: 7-8 per 10 μm (center), 10-11 (extremities).
Puncta density: 11 per 10 μm.
Length 213-225 µm, width 41-44 µm.
Stigmata visible near the central nodule.
According to Diatoms of Europe vol.3 by Kurt Krammer, 2002, it looks like Cymbella peraspera:
“Valves moderately to distinctly dorsiventral, dorsal margin rather evenly arched, ventral margin with a slightly gibbous central portion. Valve ends not protracted and broadly rounded. Length (130)154-320 µm, breadth 44-52 µm, maximal length/breadth ratio about 6. Axial area moderately wide, linear, widening at mid-valve to form a shallow central area, about ¼ to nearly ⅓ of the valve breadth. Raphe slightly lateral, tape ring near proximal and distal ends, becoming filiform near the proximal and the distal ends. Proximal raphe ends with moderately large roundish central pores which are slightly ventrally deflected; terminal fissures sickle-shaped and dorsally bent. Striae throughout radiate. Puncta distinctly and more or less roundish in focus high and low. A large number of stigmata on the ventral side of the central nodule, in focus low differently shaped from the puncta, commonly distant from the middle ventral striae. Striae 5-8/10 µm, becoming up to 10/10 µm near the extremities. Puncta 7-10(11) in 10 µm.”
Φωτογραφίες / ήχοι
Παρατηρητής
peptolabΠεριγραφή
Brachonella contorta from a four month old sample taken in August 2022 from the northernmost edge benthos of a spring-fed freshwater coastal pond in the Atlantic Double Dune Reserve. The sampling site is 250 meters from the edge of the Atlantic Ocean. They measure up to 123 um in length.
"Brachonella contorta (Levander, 1894) Jankowski, 1964 (original combination Metopus contortus Levander, 1894) Improved diagnosis based on our populations and the original description (Levander 1894): Body size about 70 − 126 × 42 − 106 um in vivo. Body bullet-shaped, massive conical preoral dome nearly obscures short bluntly truncate postoral part. Dorsoventral flattening of preoral dome rather variable. Cytostome markedly displaced posteriorly. Dense brownish-black cytoplasmic granule aggregate at the anterior end of preoral dome invariably present. Macronucleus globular, in anterior body half. Usually about 55 ciliary rows, about 22 of which are preoral dome kineties abutting to form apical suture in ventral view. Elongated evenly distributed cilia encircle posterior end. Perizonal ciliary stripe invariably comprises five rows, never arranged in false kineties, same length as adoral zone proximally, slightly longer distally. Paroral stichomonad. Adoral zone makes 360◦ spiral around long axis, usually comprises about 60 membranelles" (1).
- William Bourland, Johana Rotterová, Ivan Čepička. Redescription and molecular phylogeny of the type species for two main metopid genera, Metopus es (Müller, 1776) Lauterborn, 1916 and Brachonella contorta (Levander, 1894) Jankowski, 1964 (Metopida, Ciliophora), based on broad geographic sampling. Eur. J. Protistol. (2016), http://dx.doi.org/10.1016/j.ejop.2016.11.002
Παρατηρητής
plingfactoryΠεριγραφή
Holophtrya-species with symbiotic zoochlorellae. More on this here:
https://www.plingfactory.de/Science/Atlas/KennkartenProtista/01e-protista/e-Ciliata/e-source/Holophrya%20ovum.html
Παρατηρητής
djpmapferΠεριγραφή
Gliding heterotrophic euglenid, mouth not usually observed, but believed to be there.
Φωτογραφίες / ήχοι
Παρατηρητής
peptolabΠεριγραφή
Gruberia lanceolata from the intertidal benthos of marine estuary Accabonac Harbor. These two examples, measure 700 um in length preventing me from including the entire body in a picture! The slightly contractile body is tinted golden brown by innumerable rosy cortical granules. Imaged in Nomarski DIC using Olympus BH2 under SPlan 20x objective plus variable phone cropping on Samsung Galaxy S9+.
The discussion and description are excerpted from the paper referenced below.
Heterotrichea Stein, 1859 Heterotrichida Stein, 1859 Gruberiidae Shazib et al. (2014) Gruberia Kahl (1932) Gruberia lanceolata (Gruber 1884; Kahl 1932)
The class Heterotrichea Stein, 1854 currently comprises 10 families; one of them, Gruberiidae Shazib et al. 2014; includes only the genus Gruberia Kahl 1932 (Lynn 2008; Shazib et al. 2014). Initially, Gruberia was considered to be related to Spirostomum Ehrenberg, 1833 within the family Spirostomidae Stein, 1867 due to their highly contractile body and an adoral zone of membranelles covering approximately one-third to one half of body length. This hypothesis, however, was rejected based on molecular phylogenies (Boscaro et al. 2014; Fernandes et al. 2016; Modeo et al. 2006; Rosati et al. 2004; Shazib et al. 2014). In fact, Gruberia and Spirostomum are morphologically divergent, exhibiting distinct structures of the paroral membranes and mechanisms of cell contraction (Beltran 1933; Kahl 1932; Shazib et al. 2014). Specifically, the paroral membrane is fragmented in Gruberia, whereas it is continuous and posteriorly thickened in Spirostomum (Shazib et al. 2014). Moreover, the somatic ciliary rows become spiraled in Spirostomum during contraction, but remain meridionally orientated during contraction in Gruberia (Dragesco and Dragesco-Kerneis 1986; Shazib et al. 2014).
Cells in vivo 280–870 9 40–160 um in size and elongate fusiform with a tapered pointed posterior. Contracted cells are approximately half the length of extended cells, that is 150–450 9 40–95 um. It moves slowly on the bottom among sand grains. The macronucleus is moniliform and comprises 11–16 nodules, each 13–30 9 8–12 um in size. No micronucleus was observed. Somatic ciliature comprises approximately 24–38 kineties. The longitudinal somatic ciliary rows remain meridional during body contraction. Cortical granules rosy. The cytoplasm is colorless and contains many dispersed food vacuoles. Peristome is approximately 40–440 um long, occupying 1/3–1/2 of body length. Adoral zone with 115–330 membranelles. Paroral membrane fragmented in 35–50 short kineties with 2–6 pairs of dikinetids each. It extends parallel to the adoral zone of membranelles throughout its length. The adoral zone of membranelles performs a counterclockwise curvature, plunging into the oral infundibulum.
Journal of Eukaryotic Microbiology 2018, 0, 1–11 11 Pedro H. Campello-Nunesa, Noemi M. Fernandes, Franziska Szokoli, Giulio Petroni &
Ignacio D. da Silva-Neto. Morphology and Phylogenetic Position of Gruberia lanceolata
Φωτογραφίες / ήχοι
Παρατηρητής
mnold1Περιγραφή
Mag. 400x
Has the bloody color (red-orange) of Vampyrella (https://en.wikipedia.org/wiki/Vampyrella). V. lateritia is a strong contender for species, see images and description here https://arcella.nl/vampyrella-lateritia/. Moved surprisingly quickly (by axopod contraction, I think). In the last image, the observed rapid movement to the right has caused some of the lateral axopods to assume a swepted-back curvature.
- A water sample was taken on 10/21/2022, from the shore of one of the impoundments, using a 10µ dip net to enrich for microbes. Air temp. 65°F.
Φωτογραφίες / ήχοι
Παρατηρητής
mnold1Περιγραφή
Mag. 400x
I think this is Raphidocystis pallida, because of the similarity of these 4 specimens to images here https://arcella.nl/raphidocystis-pallida/. The stacking of scales against the base of some of the axopods gives them the look of tall pine trees (https://en.wikipedia.org/wiki/Centrohelid). For an interesting video that records the movement of the central ectoplasm, see https://youtu.be/VfGL6os0Icw.
- A water sample was taken on 10/21/2022, from the shore of one of the impoundments, using a 10µ dip net to enrich for microbes. Air temp. 65°F.
Παρατηρητής
mnold1Τόπος
ΙδιωτικόΠεριγραφή
Mag. 400x
Side view of M. furcata. For a front view, see https://www.inaturalist.org/observations/130932656.
- A water sample was taken on 10/21/2022, from the shore of one of the impoundments, using a 10µ dip net to enrich for microbes. Air temp. 65°F. (Sample was stored at room temperature and assayed again on 10/22/2022.)
Φωτογραφίες / ήχοι
Παρατηρητής
mnold1Περιγραφή
Mag. 400x
Similar to cyanobaceria Merismopedia, but with a more random and tesselated cell arrangement. Appears to have no mucilaginous sheath. Very cool! For comparison, see: https://m.blog.naver.com/PostView.naver?isHttpsRedirect=true&blogId=nstdaily&logNo=150037775947&view=img_8 and https://www.researchgate.net/publication/237991507_Occurrence_of_the_rare_genus_Microcrocis_P_Richter_Chroococcales_Cyanobacteria_in_a_coastal_lagoon_from_southern_Brazil. According to AlgaeBase, 2 species are found in marine and estuarine environments, M. sabulicola and M. marina, and are candidate IDs for the current specimen. https://www.algaebase.org/search/genus/detail/?genus_id=44298&-session=abv4:AC1F249F13c7d2CB6AQv2999E6DA
A shoreline water sample was taken on 8/9/2021 from a cove off the Thames River estuary (New London county, CT - brackish) using a 10 micron dip net to enrich for microorganisms.
Φωτογραφίες / ήχοι
Παρατηρητής
mnold1Περιγραφή
Mag. 100x (!-4), 400x (5-7)
Rotifer. As seen here http://cfb.unh.edu/cfbkey/html/Organisms/PRotifera/GBrachionus/brachionus_quadridentatus/brachionusquadridentatus.html and https://www.plingfactory.de/Science/Atlas/KennkartenTiere/Rotifers/01RotEng/source/Brachionus%20quadridentatus.html.
- A water sample was taken on 09/13/2022 from the shore of Long Pond using a 10µ dip net to enrich for microbes. Air temp. 71°F. (Sample was stored at room temperature and assayed again on 9/19/2022.)
Φωτογραφίες / ήχοι
Παρατηρητής
mnold1Περιγραφή
Mag. 100x (1), 400x (2&3)
Large, transparent rotifer, similar to Testudinella patina as seen here (similarly retracted into lorica) https://www.biolib.cz/en/image/id294315/. The video shows no movement except within the digestive tract https://youtu.be/eupS0zwgEhk.
- A bog water sample was taken on 3/15/2022 using a turkey baster swished among the submerged stems of sphagnum moss. (The area sampled was ~circular, only 15' across, and was dominated by sphagnum. Large trees and shrubs shaded the area; a small brook flowed from the circular impoundment.) Air temp. 50F
Φωτογραφίες / ήχοι
Παρατηρητής
b_harveyΠεριγραφή
Thinking this might be a telotroch peritrich filled with Zoochlorella.
Φωτογραφίες / ήχοι
Παρατηρητής
b_harveyΤόπος
ΙδιωτικόΠεριγραφή
I doubt this is in Choanozoa....thinking more along the lines of Bikosea
Φωτογραφίες / ήχοι
Παρατηρητής
sarahduhonΠεριγραφή
Lots of this stuff in Lake MacBride this weekend
Φωτογραφίες / ήχοι
Παρατηρητής
bdstaylorΠεριγραφή
From a pond with residual snow and ice, similar to the habitat in which Foissner collected the type species Wallackia schiffmanni. Oral structures are in the "Gonostomum pattern," inwardly displaced CV at bottom of AZM, very long and widely space caudal cirri, 2-nodule macronucleus. Ventral cirri were observed in vivo, as 2 long rows. Photographic records of the genus are extremely scarce.
Φωτογραφίες / ήχοι
Παρατηρητής
lamawebberΠεριγραφή
Asterionellopsis glacialis (F. Castracane) Round 1990
Light microscope (LM) images of the diatom Asterionellopsis glacialis (Castracane) Round 1990.
Phylum: Bacillariophycophyta, Class: Bacillariophyceae, Subclass: Urneidophycidae, Order: Rhaphoneidales, Family: Fragilariaceae. Girdle view. A cosmopolitan, sometimes highly abundant marine diatom in coastal cold to temperate waters. Heteropolar cells. Cells are attached by mucilage secreted from oval ocelli, with elongate openings, at the valve faces of the basal poles (see SEM images), forming stellate and spiral colonies. Basal end is triangular in girdle view and rounded in valve view with spines along the margin. A. glacialis has a labiate process at the valve end of the thin, slightly expanded extension (apex) (visible by SEM), it can be found near to the ocellus of the apex. Two chloroplasts found in the basal pole. Live specimens from Spanish Hills Wharf, Trincomali Channel, North Galiano Island, Southern Gulf Islands, British Columbia, Canada, April 6, 2022.
Nikon E800 with 20x Apo and 40x dry lenses. Stacked images. Thank you to Arjan van Asselt and Travis Olsen for taking the plankton sample.
References:
M.D. Guiry in Guiry, M.D. & Guiry, G.M. 2021. AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org; searched on 20 October 2021. http://www.algaebase.org/search/species/detail/?species_id=37056
Hoppenrath, M., Elbrachter, M., Drebes, G. (2009) Marine Phytoplankton, Selected microphytoplankton species from the North Sea around Helgoland and Sylt. pp. 103-104, figs 44 m-n. E. Schweizerbart’sche Verlagsbunchhandlung, Stuttgart, Germany.
Horner, R. A. (2002), A Taxonomic Guide to Some Common Marine Phytoplankton. p. 93. Biopress Ltd., Bristol.
Hasle, G.R. & Syvertsen, E.E. (1997). Marine Diatoms. In: Identifying Marine Phytoplankton. (Tomas, C.R. Eds.), pp. 241-243. San Diego: Academic Press.
F.E. Round, R.M. Crawford, D.G. Mann, 1990, The Diatoms, Biology & Morphology of the Genera, pp. 392-393. Cambridge University Press, Cambridge, UK.
Φωτογραφίες / ήχοι
Παρατηρητής
lamawebberΠεριγραφή
Thalassiosira cf. nordenskioeldii Cleve 1873
LM images of the marine diatom Thalassiosira cf. nordenskioeldii (Castracane) Hasle 1983. Phylum: Bacillariophycophyta, Subphylum: Bacillariophytina, Class: Mediophyceae, Order: Thalassiosirales, Family: Thalassiosiraceae. Girdle view. Thalassiosira nordenskioeldii cells are cylindrical to discoid and usually joined together in long chains. In girdle view T. nordenskioeldii cells appear as octagonal discs. They have a pronounced concavity at the valve center from which a thick mucilage tube connects to the centre of a neighbouring cell. Mantle edges are beveled. Long radiating mucilage threads exit from the prominent marginal strutted processes at the bend between the valve face and the mantle.
Cell diameter is recorded between 10-50 µm in diameter (Horner 2002: 29).
Abundant, especially during the spring bloom in the Trincomali Channel. Collected by a 20 µm plankton net April 6, 2022 from the Spanish Hills Wharf (N48˚ 59.688’, W123˚ 35.064’), Trincomali Channel, Galiano Island, Southern Gulf Islands, British Columbia, Canada.
Imaging with a Nikon E800, 20x Apo NA 0.75 and a MU2003 BI 20 MP camera and adjusted in PhotoShop.
Thank you Arjan van Asselt and Travis Olsen for collecting the sample and Travis for co-imaging.
References:
Cupp, E. E. 1943. Marine Plankton Diatoms of the West Coast of North America. University of California Press. Berkeley, California.
Hoppenrath, M., Elbrachter, M., Drebes, G. (2009) Marine Phytoplankton, Selected microphytoplankton species from the North Sea around Helgoland and Sylt. pp. 59, figure 5. Plate 24, page 58:fig. f-k. E. Schweizerbart’sche Verlagsbunchhandlung, Stuttgart, Germany.
Horner, R. A. (2002), A Taxonomic Guide to Some Common Marine Phytoplankton. p. 31. Biopress Ltd., Bristol.
Hasle, G.R. & Syvertsen, E.E. (1996). Marine Diatoms. In: Identifying Marine Phytoplankton. (Tomas, C.R. Eds). San Diego: Academic Press.
Guiry, M.D. & Guiry, G.M. 2007, AlgaeBase version 4.2. World-wide electronic publication, National University of Ireland, http://algaebase.org, searched April 10, 2022.
F.E. Round, F.E., Crawford, R.M. and Mann, D.G. (1990). The Diatoms, Biology & Morphology of the Genera. Cambridge University Press, Cambridge, UK. pp. 132-133.
Fryxell, G.A. and Hasle, G.R. (1979). The genus Thalassiosira: species with internal extensions of the strutted processes. Phycologia. vol. 18 (4), 378-393.
Pienitz, R., Fedje, D. and Poulin, M. (2003) Marine and Non-Marine Diatoms from the Haida Gwaii Archipelago and Surrounding Coasts, Northeastern Pacific, Canada In Bibliotheca Diatomologica (H. Lange-Bertalot and P. Kociolek, eds.), Band 48, J. Cramer, Stuttgart, 146 pp.
Shim, J. H. (1976). Distribution and Taxonomy of Planktonic Marine Diatoms in the Strait of Georgia, B.C. Phd. Thesis, UBC. Plate VII, fig. 2a & b.
Waters, R. E., Brown, L.N., and MG Robinson, M.G. (1992). Phytoplankton of Esquimalt Lagoon, British Columbia: comparison with west Vancouver Island coastal and offshore waters. Canadian Technical Report of Hydrography Ocean Sciences 137.
Φωτογραφίες / ήχοι
Παρατηρητής
mnold1Περιγραφή
Mag. 400x
Large Entomoneis (~120µ) comparable to images here https://diatoms.org/species/entomoneis_alata.
A shoreline water sample was taken on 8/26/2021 from a cove off the Thames River estuary (New London county, CT - brackish) using a 10 micron dip net to enrich for microorganisms.
Φωτογραφίες / ήχοι
Παρατηρητής
mnold1Περιγραφή
Mag. 400x
A shoreline water sample was taken on 8/26/2021 from a cove off the Thames River estuary (New London county, CT - brackish) using a 10 micron dip net to enrich for microorganisms.
Παρατηρητής
kenkΠεριγραφή
Time 10:00 AM
Weather: Overcast
Air temp: 12C
Water Temp: 12C
Salinity: 35ppt.
Wind: 5-10 SE
Tide: Approximately 2.1 ft. Outgoing
Secchi depth: 310 cm