Παρατηρητής
geraldojprΠεριγραφή
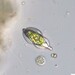
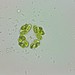
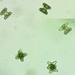
Mateola is an endemic desmid genus from the Tropical region of American continent
Παρατηρητής
geraldojprΤόπος
Rua Marquês de Paranaguá nº200 - caixa 209 - Centro, Ilhéus - BA, 45653-020, Brasil (Google, OSM)Περιγραφή
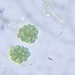
A Tropical variety of Micrasterias torreyi
Φωτογραφίες / ήχοι
Παρατηρητής
gabomagsanΠεριγραφή
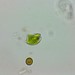
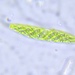
Proliferaron en una muestra de musgo sumergido en agua
Φωτογραφίες / ήχοι
Παρατηρητής
shanesmicroscopeΠεριγραφή
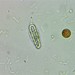
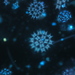
Specimen rolled over partway through the observation, allowing a nice observation of two aspects.
Παρατηρητής
kenkΠεριγραφή
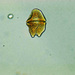
Marine sample gathered with a 20µm net.
Time 10:15 AM
Weather: Clear, Sunny
Air temp: 11C
Water Temp: 9C
Salinity: 35ppt.
Wind: 0-10 NE
Tide: Approximately 2.0 ft. Incoming
Secchi depth: 340 cm
Φωτογραφίες / ήχοι
Παρατηρητής
kenkΠεριγραφή
Reproduction by budding, or is it division?
Time 9:40 AM
Weather: Cloudy
Air temp: 10C
Water Temp: 12C
Salinity: 33ppt.
Wind: 0-5 MPH, NW
Tide: High tide, 6.67ft. (King tide)
Secchi depth: 340 cm
Φωτογραφίες / ήχοι
Παρατηρητής
mnold1Περιγραφή
Mag. 400x
Longish (200µ). U. piscis? Looks like 3 rows of ventral cirri... diagnostic?
@bdstaylor, @shanesmicroscope, @crseaquist
- A pond-side water sample was taken on 12/27/2021 using a 10µ dip net. About 1/4" of ice had to be broken and pushed aside to access the water. Air temp. 36°F.
Φωτογραφίες / ήχοι
Παρατηρητής
mnold1Περιγραφή
Mag. 400x (1), 100x (2,3)
Somewhat evenly spaced clusters of yellow-brown cells on a strand of Eunotia sp. cells. Algal epiphytes? Egg deposits?
Φωτογραφίες / ήχοι
Τι
Ζωή (Ζωή)Παρατηρητής
mnold1Περιγραφή
Mag. 400x (freshwater)
Uniformly oval. Regularly spaced spikes. There is also a smaller spike-covered object (rod shaped?), just behind or in front of the large oval. (I can't tell where the focal plane is sitting.) The smaller object is visible in the lower panels of the photo composite, image 2. If the primary object is an egg case, what is the smaller spiky object? Perhaps the smaller object is a peal projecting from the large oval...like projecting panel from an opening chestnut. Interesting!
- A pond-side water sample was taken on 12/27/2021 using a 10µ dip net. About 1/4" of ice had to be broken and pushed aside to access the water. Air temp. 36°F.
Φωτογραφίες / ήχοι
Παρατηρητής
mnold1Περιγραφή
Mag. 400x
13 dorsal margin undulation. As seen here https://diatoms.org/species/eunotia_serra. (note: this taxa can have 6-22 dorsal margin undulations)
- A pond edge water sample (freshwater) was taken on 2/9/2022 using a 10 micron dip net to enrich for microorganisms. Air temp. 41C. Sampling area was free of ice due the water flow through a nearby culvert.
Φωτογραφίες / ήχοι
Παρατηρητής
mnold1Περιγραφή
Mag. 400x (1,2), 100x (3)
- Estuarine environment (brackish). A river-side water sample was taken on 1/27/2022 using a large plastic cup to scoop rocks, organic debris, ice and water (no sand or soil). The settled material was retained along with some of the water. Air temp. 30°F.
Φωτογραφίες / ήχοι
Παρατηρητής
kenkΠεριγραφή
Time 3:30 PM
Weather: Clear
Air temp: 12C
Water Temp: 8.5C
Salinity: 34ppt.
Wind: 10-20 MPH, NNE
Tide: High tide 2.95
Secchi depth: 340cm
Φωτογραφίες / ήχοι
Παρατηρητής
crseaquistΠεριγραφή
A stream water sample (freshwater) was taken on 11/06/2021 using a 10 micron dip net to enrich for microorganisms.
Moved 50-100µm in less than a minute.
Φωτογραφίες / ήχοι
Παρατηρητής
shanesmicroscopeΠεριγραφή
Philodina laying an egg. For some reason the Dexiotricha were very interested in the proceedings.
Φωτογραφίες / ήχοι
Παρατηρητής
crseaquistΠεριγραφή
A pond edge water sample (freshwater) was taken on 10/19/2021 using a 10 micron dip net to enrich for microorganisms.
Φωτογραφίες / ήχοι
Παρατηρητής
mnold1Περιγραφή
Mag. 400x
Each spine is rooted in a circular scale (photo 2). Circular egg (photo 4)?
A pond edge water sample (freshwater) was taken on 10/14/2021 using a 10 micron dip net to enrich for microorganisms.
Παρατηρητής
louiselewisΠεριγραφή
Collected from a freshwater pool in the Ecology and Evolutionary Biology Greenhouse.
Φωτογραφίες / ήχοι
Παρατηρητής
mnold1Περιγραφή
Mag. 400x (1, 4gif,6), 100x (2,5), 25x (3)
By far (>95%), this was the predominant specie netted during this collection... a bloom of C. longispina. Each cell has several, long siliceous spines and 2 flagella (one short, one long) http://www.diatom.org/lakes/taxa/chryso/Chrysosphaerella/TB-Chrysosphaerella_longispina.htm. Image 3 is a gif; careful inspection will show flagellar movement. -- The slide was allowed to dry and then reexamined after several days, see image 5. The drying process appears to have released some of the spines from the cells. Their double-headed nail-like structure is apparent in image 6 (also see http://cfb.unh.edu/phycokey/Choices/Synurophyceae/CHRYSOSPHAERELLA/Chrysosphaerella_Image_page.html)
A pond edge water sample (freshwater) was taken on 10/14/2021 using a 10 micron dip net to enrich for microorganisms.
Φωτογραφίες / ήχοι
Παρατηρητής
shanesmicroscopeΗμερομηνία
Σεπτέμβριος 2021Περιγραφή
A sample of seaweed collected from the beach at Ocean City, NJ.
Φωτογραφίες / ήχοι
Παρατηρητής
mnold1Περιγραφή
Mag. 400x
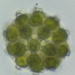
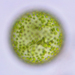
16-cell colony. Each cell is tethered to a single stalk that converges, by tetrachotomous branching, in the center of the colony; there are 4 cells at the end of each major branchlet. The bottom frame in the composite photo (last photo) best shows the branching system. Compare to images here https://www.algaebase.org/search/species/detail/?species_id=176802&sk=0&from=results.
A pond edge water sample (freshwater) was taken on 10/1/2021 using a 10 micron dip net to enrich for microorganisms.